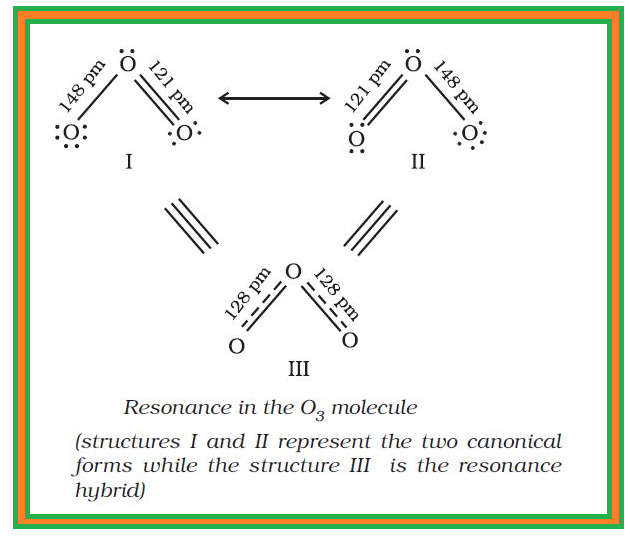
`=>` The existence of a hundred percent ionic or covalent bond represents an ideal situation.
`=>` But, in reality no bond or a compound is either completely covalent or ionic.
● Even in case of covalent bond between two hydrogen atoms, there is some ionic character.
`=>` When covalent bond is formed between two similar atoms, for example in `H_2`, `O_2`, `Cl_2`, `N_2` or `F_2,` the shared pair of electrons is equally attracted by the two atoms.
● As a result electron pair is situated exactly between the two identical nuclei. The bond so formed is called `color{red}("non-polar covalent bond")`.
`=>` In case of a heteronuclear molecule like `HF`, the shared electron pair between the two atoms gets displaced more towards fluorine since the electronegativity of fluorine is far greater than that of hydrogen. The resultant covalent bond is a `color{red}("polar covalent bond")`.
`=>` As a result of polarisation, the molecule possesses the `color{red}("dipole moment")` which can be defined as the product of the magnitude of the charge and the distance between the centres of positive and negative charge.
● It is usually designated by a Greek letter ‘μ’.
● Mathematically, it is expressed as follows :
● Dipole moment (`μ`) = charge (`Q`) × distance of separation (`r`)
● Dipole moment is usually expressed in Debye units (`D`).
● The conversion factor is
`1 D = 3.33564 × 10^(–30) C m`
where `C` is coulomb and `m` is meter.
● Further dipole moment is a vector quantity and is depicted by a small arrow with tail on the positive centre and head pointing towards the negative centre.
● For example the dipole moment of `HF` may be represented as :
`underset{H - overset(. . )F : } ↛`
● The shift in electron density is symbolised by crossed arrow `↛ ` above the Lewis structure to indicate the direction of the shift.
`=>` In case of polyatomic molecules the dipole moment not only depend upon the individual dipole moments of bonds known as bond dipoles but also on the spatial arrangement of various bonds in the molecule. In such case, the dipole moment of a molecule is the vector sum of the dipole moments of various bonds.
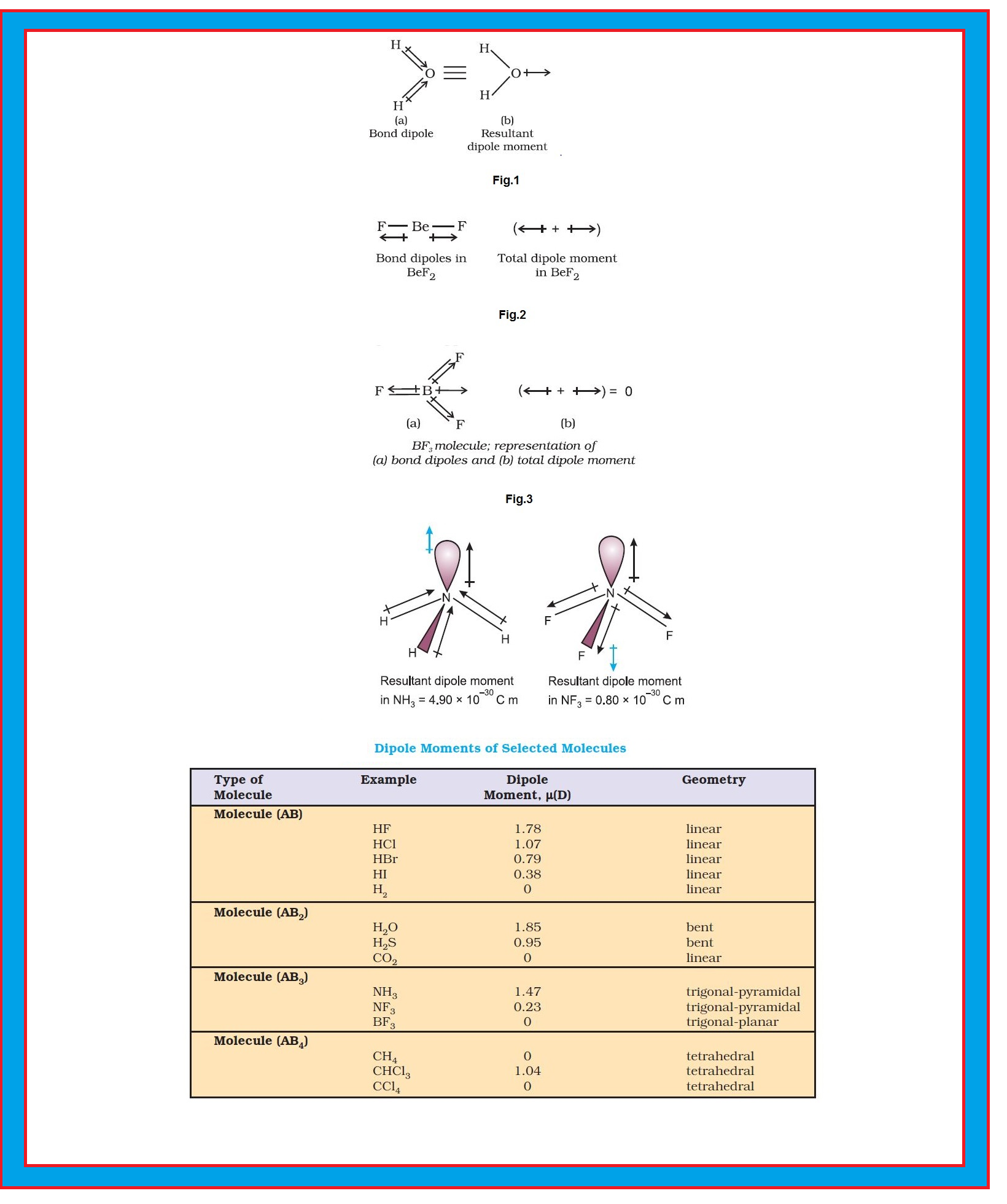
`color{red}("Example ")` (i) In `H_2O` molecule, which has a bent structure, the two `O–H` bonds are oriented at an angle of `104.5^0`. Net dipole moment of `6.17 × 10^(–30) C m (1D = 3.33564 × 10^(–30) C m)` is the resultant of the dipole moments of two `O–H` bonds. See fig.1.
● Net Dipole moment, `μ = 1.85 D = 1.85 × 3.33564 × 10^(–30) C m = 6.17 ×10^(–30) C m`
(ii) The dipole moment in case of `BeF_2` is zero. This is because the two equal bond dipoles point in opposite directions and cancel the
effect of each other. See fig.2.
(iii) In tetra-atomic molecule, for example in `BF_3`, the dipole moment is zero although the `B – F` bonds are oriented at an angle of `120°` to one another, the three bond moments give a net sum of zero as the resultant of any two is equal and opposite to the third. See fig.3.
(iv) Let's study about `NH_3` and `NF_3` molecule.
● Both the molecules have pyramidal shape with a lone pair of electrons on nitrogen atom.
● Although fluorine is more electronegative than nitrogen, the resultant dipole moment of `NH_3 ( 4.90 × 10^(–30) C m)` is greater than that of `NF_3 (0.8 × 10^(–30) C m)`.
● This is because, in case of `NH_3` the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the `N – H` bonds, whereas in `NF_3` the orbital dipole is in the direction opposite to the resultant dipole moment of the three `N–F` bonds.
● The orbital dipole because of lone pair decreases the effect of the resultant `N – F` bond moments, which results in the low dipole moment of `NF_3` as represented in fig.4.
`=>` Dipole moments of some molecules are shown in Table 4.5.
`=>` Just as all the covalent bonds have some partial ionic character, the ionic bonds also have partial covalent character.
`color{green}("Fajan's Rule ")`
`=>` The partial covalent character of ionic bonds was discussed by Fajans in terms of the following rules :
● The smaller the size of the cation and the larger the size of the anion, the greater the covalent character of an ionic bond.
● The greater the charge on the cation, the greater the covalent character of the ionic bond.
● For cations of the same size and charge, the one, with electronic configuration `(n-1)d^n ns^0`, typical of transition metals, is more polarising than the one with a noble gas configuration, `ns^2 np^6`, typical of alkali and alkaline earth metal cations.
`=>` The cation polarises the anion, pulling the electronic charge toward itself and thereby increasing the electronic charge between the two. This is precisely what happens in a covalent bond, i.e., buildup of electron charge density between the nuclei.
`=>` The polarising power of the cation, the polarisability of the anion and the extent of distortion (polarisation) of anion are the factors, which determine the per cent covalent character of the ionic bond.
`=>` The existence of a hundred percent ionic or covalent bond represents an ideal situation.
`=>` But, in reality no bond or a compound is either completely covalent or ionic.
● Even in case of covalent bond between two hydrogen atoms, there is some ionic character.
`=>` When covalent bond is formed between two similar atoms, for example in `H_2`, `O_2`, `Cl_2`, `N_2` or `F_2,` the shared pair of electrons is equally attracted by the two atoms.
● As a result electron pair is situated exactly between the two identical nuclei. The bond so formed is called `color{red}("non-polar covalent bond")`.
`=>` In case of a heteronuclear molecule like `HF`, the shared electron pair between the two atoms gets displaced more towards fluorine since the electronegativity of fluorine is far greater than that of hydrogen. The resultant covalent bond is a `color{red}("polar covalent bond")`.
`=>` As a result of polarisation, the molecule possesses the `color{red}("dipole moment")` which can be defined as the product of the magnitude of the charge and the distance between the centres of positive and negative charge.
● It is usually designated by a Greek letter ‘μ’.
● Mathematically, it is expressed as follows :
● Dipole moment (`μ`) = charge (`Q`) × distance of separation (`r`)
● Dipole moment is usually expressed in Debye units (`D`).
● The conversion factor is
`1 D = 3.33564 × 10^(–30) C m`
where `C` is coulomb and `m` is meter.
● Further dipole moment is a vector quantity and is depicted by a small arrow with tail on the positive centre and head pointing towards the negative centre.
● For example the dipole moment of `HF` may be represented as :
`underset{H - overset(. . )F : } ↛`
● The shift in electron density is symbolised by crossed arrow `↛ ` above the Lewis structure to indicate the direction of the shift.
`=>` In case of polyatomic molecules the dipole moment not only depend upon the individual dipole moments of bonds known as bond dipoles but also on the spatial arrangement of various bonds in the molecule. In such case, the dipole moment of a molecule is the vector sum of the dipole moments of various bonds.
`color{red}("Example ")` (i) In `H_2O` molecule, which has a bent structure, the two `O–H` bonds are oriented at an angle of `104.5^0`. Net dipole moment of `6.17 × 10^(–30) C m (1D = 3.33564 × 10^(–30) C m)` is the resultant of the dipole moments of two `O–H` bonds. See fig.1.
● Net Dipole moment, `μ = 1.85 D = 1.85 × 3.33564 × 10^(–30) C m = 6.17 ×10^(–30) C m`
(ii) The dipole moment in case of `BeF_2` is zero. This is because the two equal bond dipoles point in opposite directions and cancel the
effect of each other. See fig.2.
(iii) In tetra-atomic molecule, for example in `BF_3`, the dipole moment is zero although the `B – F` bonds are oriented at an angle of `120°` to one another, the three bond moments give a net sum of zero as the resultant of any two is equal and opposite to the third. See fig.3.
(iv) Let's study about `NH_3` and `NF_3` molecule.
● Both the molecules have pyramidal shape with a lone pair of electrons on nitrogen atom.
● Although fluorine is more electronegative than nitrogen, the resultant dipole moment of `NH_3 ( 4.90 × 10^(–30) C m)` is greater than that of `NF_3 (0.8 × 10^(–30) C m)`.
● This is because, in case of `NH_3` the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the `N – H` bonds, whereas in `NF_3` the orbital dipole is in the direction opposite to the resultant dipole moment of the three `N–F` bonds.
● The orbital dipole because of lone pair decreases the effect of the resultant `N – F` bond moments, which results in the low dipole moment of `NF_3` as represented in fig.4.
`=>` Dipole moments of some molecules are shown in Table 4.5.
`=>` Just as all the covalent bonds have some partial ionic character, the ionic bonds also have partial covalent character.
`color{green}("Fajan's Rule ")`
`=>` The partial covalent character of ionic bonds was discussed by Fajans in terms of the following rules :
● The smaller the size of the cation and the larger the size of the anion, the greater the covalent character of an ionic bond.
● The greater the charge on the cation, the greater the covalent character of the ionic bond.
● For cations of the same size and charge, the one, with electronic configuration `(n-1)d^n ns^0`, typical of transition metals, is more polarising than the one with a noble gas configuration, `ns^2 np^6`, typical of alkali and alkaline earth metal cations.
`=>` The cation polarises the anion, pulling the electronic charge toward itself and thereby increasing the electronic charge between the two. This is precisely what happens in a covalent bond, i.e., buildup of electron charge density between the nuclei.
`=>` The polarising power of the cation, the polarisability of the anion and the extent of distortion (polarisation) of anion are the factors, which determine the per cent covalent character of the ionic bond.